Imagine a future where cancer patients receive personalized treatments tailored to their genetic makeup. Not only is this possible today, but it’s just one of several biotech breakthroughs stemming from giant leaps in science. These innovations are sending waves through the drug product market and streamlining regulatory frameworks.
To maintain their ability to innovate in their search for tomorrow’s cures, drugmakers are updating their facilities across the spectrum of the drug product delivery pipeline—from research to product distribution. This requires continuous updates to their teams, tech, processes and buildings. Central to this is the critical reassessment and adaptation of their cGMP (current Good Manufacturing Practice) facilities. They must be able to respond to these trends and unforeseen challenges still to come.
Depending on where a company falls on the spectrum of biotech startup to global pharmaceutical enterprise, it can have very different facility requirements. The key is for their facilities to support their changing priorities at each stage. To maintain productivity during transitions from one stage to the next, HOK uses an integrated facility planning approach that considers the full product life cycle.
Scientific Advances Toward Personalized Medicine
Advancements in genetics, immunology and cell biology are unlocking new possibilities for targeted, personalized therapies. The plummeting costs of human gene sequencing have enabled scientists to quickly identify treatments for specific diseases and patients. Ai and machine learning are further accelerating drug discovery and development by allowing scientists to analyze complex data and optimize therapeutic strategies. These novel treatments and immunotherapies are shifting production from traditional large-scale batches to smaller, patient-tailored offerings.
This momentum toward individualized medicine requires more flexible cGMP facilities equipped with automation and robotics to improve production speed, precision and consistency.
Additionally, augmented and virtual reality (AR/VR) technologies are revolutionizing training, maintenance and remote collaboration methods in these facilities. The use of data-driven ‘digital twin’ models is a growing trend that allows for the simulation and ongoing refinement of manufacturing processes. Digitized platforms play a crucial role, too, by interconnecting equipment, monitoring environmental conditions, tracking supply chains and more.
These rapid technological developments are fueling growth in the industry and leading to highly promising patient outcomes. To maximize the benefits of such personalized treatments and improvements in the manufacturing process, however, regulatory processes must be streamlined—a change that’s currently underway.
Adapting Regulations to Innovation

Responding to the demands of healthcare professionals and the unprecedented pace of scientific innovation in the life science industry, the U.S. Food and Drug Administration (FDA) and global regulators are expediting their review and approval timelines. The FDA created designations like Breakthrough Therapy and Regenerative Medicine Advanced Therapy (RMAT) to slash the traditionally lengthy clinical trial approval pathways for new treatments.
The U.S. Congressional Budget Office reports a significant increase in drug approvals: “On average, [the FDA] approved 38 new drugs per year from 2010 through 2019 (with a peak of 59 in 2018), which is 60 percent more than the yearly average over the previous decade.” In 2023, the FDA approved 55 novel drugs.
Market Forces Driving Trends

Though regulatory changes are facilitating progress, they can’t fully address the surge in new therapies on the horizon. The noticeable increase in small biotech firms and their frequent acquisitions by larger pharmaceutical companies signal a focus on filling product pipelines with promising treatments. After discovering a successful drug, these companies face intense pressure to deliver it to patients quickly. This urgency creates a need to speed up the clinical trial and FDA approval process of transitioning new products to commercial manufacturing.
Amid these shifts, Contract Development and Manufacturing Organizations (CDMOs) are playing a bigger role. Experts estimate the bio and pharma CDMO sector, currently valued at $223 billion, will expand to $309 billion by 2028. This reflects the industry’s growing dependence on external partners for flexible, scalable solutions catering to emerging startups and established companies.
Startup and Growth

In a startup’s early stages, teams are small and seed money is limited. To stretch these dollars, we minimize the leased footprint by designing flexible spaces that drive innovation and collaboration.
Startups often emerge from an academic environment before expanding into speculative lab leases of research incubators. We anticipate that space needs will change often, and lay out their labs with open plans and modular benches that provide flexibility for quick iteration and refinement of different therapies. When it is essential, we include specialty equipment rooms. Our approach gives users a variety of adaptable multi-use spaces that maximize functionality by using movable furniture, including writing surfaces, integrating technology and considering acoustic issues like sound transfer and speech intelligibility. To boost interaction and collaboration, we provide open office designs and shared common spaces that accommodate expansion for evolving staffing needs.
When the research yields promising results, startups may seek financing to pilot test products at a small scale. Not only does this next stage of growth increase demand for office and collaboration spaces, but research facility needs also grow. Pilot plants are used to test small batches and conduct early clinical trials. These cleanroom environments have gowning facilities, airlocks, storage rooms, equipment rooms and quality control labs. By creating clear diagrams of the personnel and material flows, identifying gowning requirements and defining airlock configurations and airflow cascades, we help our clients mitigate contamination risks, which aids in the validation and facility approval process.
At a confidential company’s pilot facility in Maryland, researchers are pioneering groundbreaking technologies to produce new medicines. To support the company’s Phase 1 and 2 clinical trials on this campus, HOK designed a cell therapy suite meeting U.S. and E.U. regulations and an antibody drug conjugate (ADC) pilot manufacturing facility.
Expansion and Maturity

As life science companies transition from R&D to commercialization, their manufacturing facilities must scale up production capacity. This requires major capital investments that may exceed available funding and delay their growth plans—even if product development stays on schedule.
An imminent FDA approval can thrust an organization into high gear. Its facility needs expand to include large-scale production and warehousing, often requiring significant capital infusions. At this stage, companies may go public, seek private investment or partner with larger corporations to expand.
To support this growth to large-scale production and commercialization, designers must plan for an array of support spaces in an FDA-validated, market launch-ready facility. These include:
- Receiving and storage
- Sample processing and prep areas
- Production and processing zones
- Cleaning and sterilization facilities
- Finishing and distribution centers
Once a program has been established, we create a master plan that evaluates factors like utility infrastructure, transportation access, expansion planning, manufacturing speed and capacity, and environmental risks.
NextGen (continuous) manufacturing is a process that generates more material, faster, using smaller equipment and facilities, with a smaller environmental footprint and a reduced contamination risk. Thoughtful planning incorporates these process engineering efficiencies early in the schedule and leads to conversations about phasing and expansion. As illustrated in the diagram below, facility expansion off a shared central corridor maintains the operation of existing cell therapy suites and provides shared use of common facilities like the consumable warehouse, QC labs and the existing entry points from gowning.
We seek to mitigate risks from climate events or equipment failure by developing a facility resiliency plan and identifying single points of failure. This ensures the site can sustain long-term operations while enabling optimal functionality amidst external surprises through every stage of development.


10x Genomics’ new lab and office building (above) in Pleasanton, California, is the first phase of a three-building “science village” campus. Emphasizing flexibility and scalability, our design accommodates the evolving requirements of modern biotechnology. Shared gowning areas, modular cleanrooms and advanced dispensing labs ensure the facility can adapt to 10x Genomics’ growth.
To optimize flexibility and adaptability in these buildings, we compartmentalize spaces to support scaling production, phasing construction and easily repurposing areas as processes change. This strategic planning builds resilience while generating long-term cost efficiencies.
Common flexibility strategies for accommodating reconfiguration and growth with limited downtime include:
- Standardized mobile furnishings
- Overhead utilities
- Distributed shell space for phased expansion
- Accessible maintenance service space
- Modular cleanroom pods to streamline fit-outs
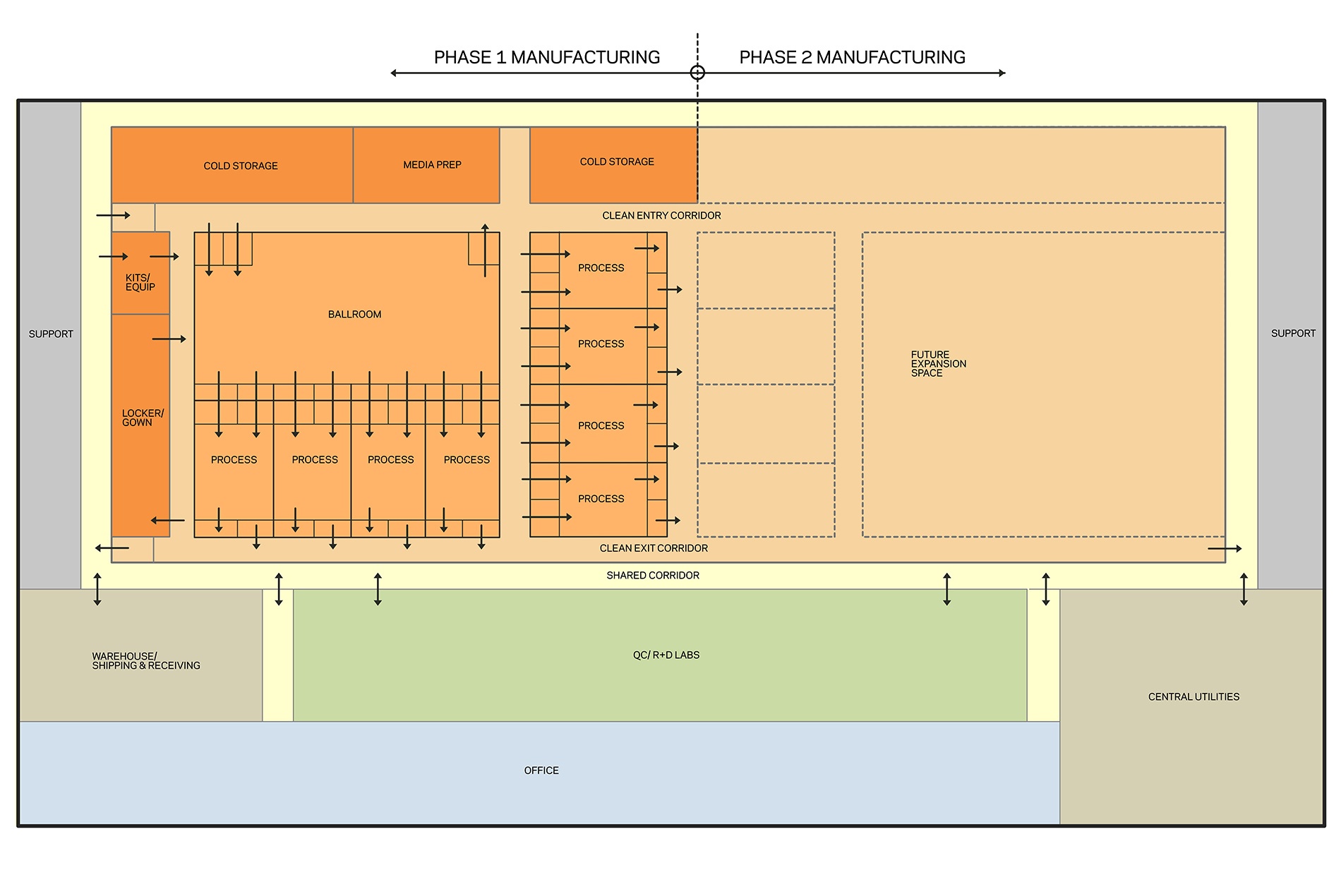
HOK designed a cell therapy manufacturing facility (above) for a confidential commercial research organization. Here, our team planned multi-product process suites to contain products in development, including viral vector platforms. The process zone consisted of two cores, each with a ballroom for upstream processing and a flexible, smaller process for downstream or independent workflows.
Incorporating Sustainability and Resiliency

With their high energy and water demands, research and manufacturing facilities offer significant opportunities for integrating sustainable design strategies. Airflow in cleanroom environments and pure water systems are both major energy drivers in pilot and cGMP facilities.
Manufacturing facilities are designed with different cleanroom classifications. The more a drug substance is exposed to the air, the lower the classification number and the more air changes are required to clean the air. Closed-process, single-use systems can significantly reduce cleanroom airflow requirements and the associated power consumption. The single-use systems enable higher classifications and less airflow in more environments. This reduces air handler sizes, equipment room sizes and overall power usage, resulting in significant embodied and operational carbon reductions.
Single-use systems also replace the need for open systems that require on-site cleaning and sterilization, thereby reducing the space needed for glass washing and sterilization equipment and the energy they consume.
Water for injection (WFI) is a pure water system that is historically an energy-intense process utility. The system requires energy to continuously heat water for sterilization, and more power to cool the water down to ambient temperatures. Strategies for “Cold WFI” generation and ozone sterilization, coupled with single-use products, can reduce the need for process steam and even eliminate the need for a plant steam system, opening the possibility of a carbon-free facility.
Though it was not a project HOK worked on, Affiliated Engineers, Inc. designed the Agenus cGMP facility in Emeryville, California, based on this model, with a production capacity of 4 x 2,000 liters.
People First

We have already described how designers of cGMP facilities must address elements like therapy type, manufacturing scale, growth stage and flexibility. Along with these considerations, the most successful cGMP facilities prioritize one aspect above all else—the people who work there.
At HOK, we start by crafting a human-centered design that:
- Reflects the clients’ mission and culture
- Incorporates sustainability
- Promotes equity and community
Reflecting Mission and Culture
Collaborating with our clients, we gather data to understand their ethos and values then translate them into the design of their facilities. As products and teams scale, we consider how new spaces will support the organization’s culture.
One past client of mine established a custom of taking puzzle breaks to reset its team’s focus. As this organization grew, our design team honored this tradition by ensuring its facilities always included puzzle tables.
This is just one example of how designers can customize spaces to resonate with an organization. Doing this strengthens employee engagement and retention while helping new employees connect with the company’s unique culture. In key amenity areas, we harness bold forms, environmental graphics, furniture, materials and artwork as opportunities to strengthen the expression of company values and cultural ties.
For AstraZeneca’s Lab and Office Facility in South San Francisco (above), HOK designed a research facility that fits the company’s culture while creating a highly collaborative research environment. The design maximizes transparency across lab, office and public spaces. Central spaces in the center of each floor serve as employee hubs. Designed with a focus on sustainability, the project earned LEED Platinum certification.
For more insights into trends in scientific workplace design, refer to HOK’s article, “Trends in the Scientific Workplace: The Shape of Labs to Come.”
Promoting Equity and Community
Partnering with local stakeholders and diverse businesses in the design process can have many positive impacts. In our experience, these collaborations promote innovation and benefit surrounding communities by creating jobs, educational programs and civic spaces. We work closely with owners and local design and construction partners to develop public amenities like cafes, auditoriums, fitness centers or multi-use spaces. These amenities can be harnessed to strengthen community ties and build trust among stakeholders.
For HOK’s interior design of AstraZeneca’s new R&D site in Cambridge, Massachusetts, our team embedded diversity, equity and inclusion into every facet of the design process. Engaging AstraZeneca’s internal DEI stakeholders in visioning sessions gave our team insights that shaped the building’s spaces to accommodate many different needs.
Let's Connect
Scientific innovation is driving the development of more targeted, personalized therapies. To keep pace, research and manufacturing facilities need flexible, adaptable spaces that can easily scale up.
As startups transition into global enterprises, their facilities must support changing priorities at each stage. HOK’s human-centered design approach focuses on understanding our clients’ mission and culture. We translate what we learn into spaces tailored to their specific needs, always balancing customization with standardization. By combining strategic modular planning with deep technical expertise, we create facilities that are ready for the future.
The result? Environments equipped to help translate innovative research into life-enhancing and lifesaving solutions.
Planning a new or upgraded research or manufacturing facility? Contact Dan Seng, AIA, CDT, LEED AP BD+C, HOK’s practice leader of Science + Technology in Seattle, at dan.seng@hok.com.
